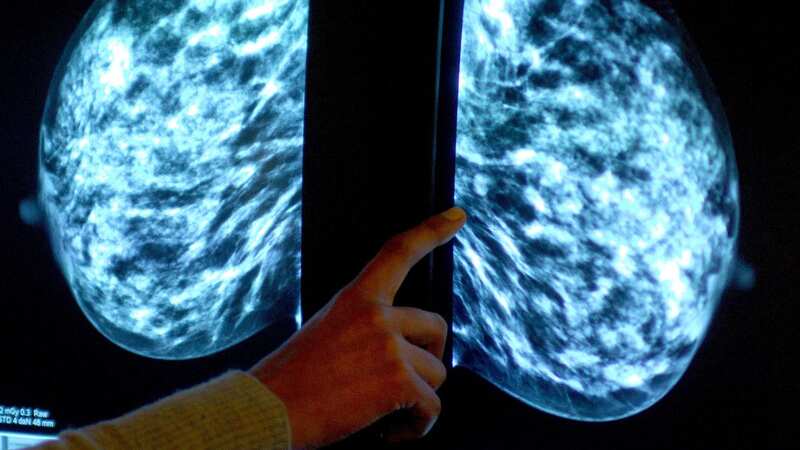

A treatment that extends the life of people with advanced breast cancer has been blocked for NHS use in England in a move that potentially impacts thousands of patients.
The National Institute for Health and Care Excellence (Nice) said the cost the NHS was being asked to pay for trastuzumab deruxtecan – sold under the brand name Enhertu – was "too high" in relation to its benefits. The charity Breast Cancer Now has described the decision as a "dark day" for patients with the disease and urged manufacturer Daiichi Sankyo, which developed the drug with AstraZeneca, to "come back to the table" with Nice and NHS England.
Enhertu is the first licensed targeted treatment for patients with HER2-low breast cancer that cannot be removed surgically or that has spread to other parts of the body, also known as metastatic breast cancer. Patients are offered treatments for HER2-negative cancer – usually chemotherapy – but could benefit from targeted therapies.
Draft guidance published by Nice in September said it would not recommend Enhertu for NHS use in England due to uncertainties in the information provided by the manufacturer and called for more details. The spending watchdog then paused its appraisal in December while commercial talks were ongoing, but they have now concluded without a price agreement in place.
Had Nice recommended the treatment, about 1,000 patients a year would have been eligible. Baroness Delyth Morgan, chief executive at Breast Cancer Now, said: "This is a dark day for people affected by incurable secondary breast cancer. NHS England, Nice, Daiichi Sankyo and AstraZeneca have failed people living with the disease. They've blocked a vital treatment that offers hope of more time to live for thousands of people with a certain type of secondary breast cancer."
 Teachers, civil servants and train drivers walk out in biggest strike in decade
Teachers, civil servants and train drivers walk out in biggest strike in decade
Helen Knight, director of medicines evaluation at Nice, said the costs the health service was being asked to pay was "too high in relation to the benefits" of Enhertu and the organisation is "extremely disappointed" to not be able to recommend the treatment for NHS use.
She added: "The backdrop to the commercial discussions was the independent appraisal committee's belief that, based on the available evidence, Enhertu represents a significant development for people with HER2-low advanced breast cancer who currently have limited chemotherapy options and most have no targeted treatments available to them. However, a key uncertainty in estimating Enhertu's cost-effectiveness was how much longer people on Enhertu live compared with those receiving standard treatment in the future."
"The independent committee carefully considered all the evidence and applied its judgement on the most clinically plausible approach on which to base its decision." Enhertu was approved for use on the NHS in Scotland by the Scottish Medicines Consortium (SMC) in December.
Haran Maheson, vice president and head of oncology at Daiichi Sankyo UK, warned that patients in England will now face a "postcode lottery" for treatment. "We are extremely disappointed that patients with breast cancer in England and Wales are going to lose out on an effective treatment due to a technicality in the new formula Nice uses to assess cancer medicines," he said.
"As we have demonstrated in Scotland, it is possible to provide access to this medicine cost-effectively within the UK. Patients now face a postcode lottery. This is the first and only licenced HER2-targeted medicine effective in HER2-low breast cancer and unfortunately Nice's approach does not adequately recognise the severity of this devastating condition.
"We remain determined to find a solution to deliver equitable access to patients across the UK and urge Nice to be more constructive in exercising flexibility in its assessment process."
Baroness Morgan said the decision for England means "thousands of mums, daughters, sisters, wives, colleagues, and friends who want to be there and create special memories, now face the unbearable reality of knowing a treatment that could have been a lifeline for them exists, but remains out of reach, while women in Scotland have been granted access".
"Neither we, nor people affected by secondary breast cancer will walk away," she added. "Nice, NHS England, Daiichi Sankyo and AstraZeneca must not either – they must come back to the table, and a solution found that puts women with secondary breast cancer first.
"NHS England must do all they can to be flexible and help deliver their continued commitment to getting the latest cutting-edge drugs to patients. Daiichi Sankyo and AstraZeneca must ensure they are doing everything possible to price the drug at a cost that is fair to the NHS."
An NHS spokesperson said: "NHS England expected drug companies AstraZeneca and Daiichi Sankyo to offer this treatment at a price that would enable Nice to recommend its use for patients with secondary breast cancer. We are deeply disappointed that AstraZeneca and Daiichi Sankyo have not been willing to price this treatment to enable approval, therefore denying NHS patients the opportunity to access this latest advance in care."
 Greggs, Costa & Pret coffees have 'huge differences in caffeine', says report
Greggs, Costa & Pret coffees have 'huge differences in caffeine', says report